Delve into the fascinating world of binary covalent compounds with our comprehensive naming binary covalent compounds worksheet. This worksheet provides a step-by-step guide to understanding the principles and applications of naming these essential chemical entities.
This resource is meticulously designed to cater to students, educators, and professionals seeking to enhance their knowledge of binary covalent compounds. Its interactive exercises and detailed explanations empower learners to grasp the intricacies of this topic effortlessly.
Naming Binary Covalent Compounds: Naming Binary Covalent Compounds Worksheet
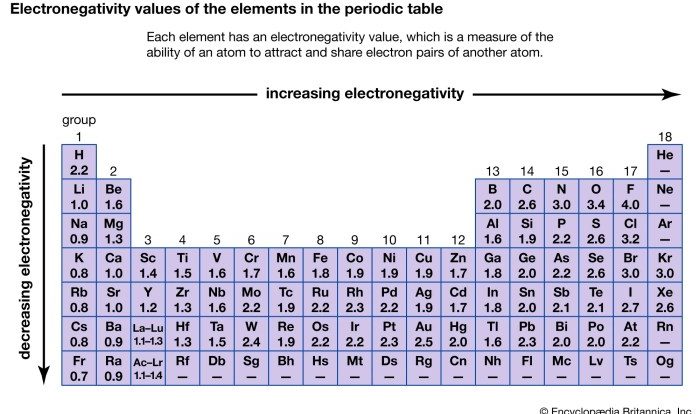
Binary covalent compounds are chemical compounds composed of two non-metal elements. They are formed when atoms of the two elements share electrons to form covalent bonds.
Examples of binary covalent compounds include:
- Water (H 2O)
- Carbon dioxide (CO 2)
- Ammonia (NH 3)
Naming Binary Covalent Compounds, Naming binary covalent compounds worksheet
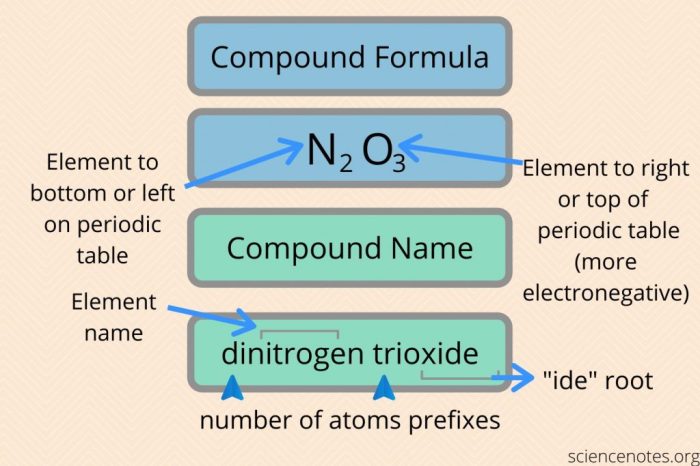
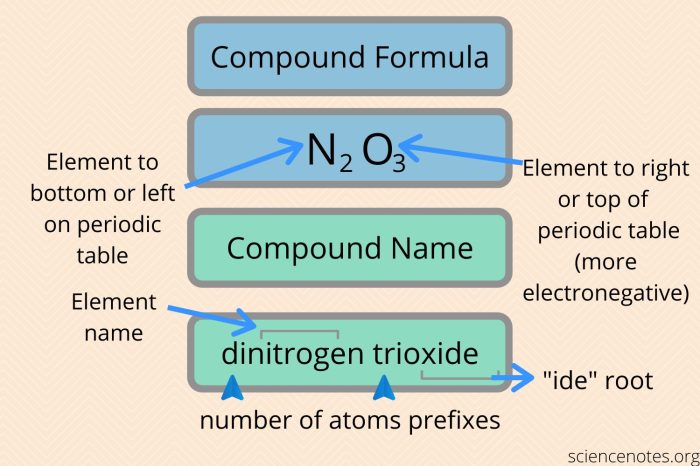
The rules for naming binary covalent compounds are as follows:
- The first element in the name is the element that is written first in the chemical formula.
- The second element in the name is the element that is written second in the chemical formula.
- The suffix -ideis added to the name of the second element.
- Prefixes are used to indicate the number of atoms of each element in the compound. The prefixes are mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, and deca-.
For example, the compound CO 2is named carbon dioxide. The first element in the name is carbon, and the second element in the name is oxygen. The suffix -ideis added to the name of oxygen. The prefix di-is used to indicate that there are two atoms of oxygen in the compound.
Worksheet
Complete the following worksheet to practice naming binary covalent compounds.
- Name the compound with the chemical formula HCl.
- Name the compound with the chemical formula N 2O 5.
- Name the compound with the chemical formula SF 6.
Answer Key
- Hydrogen chloride
- Dinitrogen pentoxide
- Sulfur hexafluoride
Examples and Applications
Binary covalent compounds are found in a wide variety of applications, including:
- Water is essential for life on Earth.
- Carbon dioxide is used as a fire extinguisher.
- Ammonia is used as a fertilizer.
The following table provides a more comprehensive list of examples and applications of binary covalent compounds:
| Compound | Example | Application |
|---|---|---|
| Water (H2O) | Rain, rivers, lakes, oceans | Essential for life on Earth |
| Carbon dioxide (CO2) | Soda water, fire extinguishers | Fire extinguisher |
| Ammonia (NH3) | Fertilizer, cleaning products | Fertilizer |
| Sodium chloride (NaCl) | Table salt, rock salt | Seasoning, food preservative |
| Hydrogen fluoride (HF) | Etching glass, cleaning metal | Etching glass |
| Silicon dioxide (SiO2) | Sand, quartz | Glass, ceramics |
Question & Answer Hub
What are binary covalent compounds?
Binary covalent compounds are chemical compounds composed of two non-metallic elements that are joined by covalent bonds.
How do I name binary covalent compounds?
To name binary covalent compounds, use the following steps: 1) Identify the elements present in the compound. 2) Write the name of the first element, followed by the root of the name of the second element. 3) Add the suffix “-ide” to the root of the second element’s name.
What are some examples of binary covalent compounds?
Examples of binary covalent compounds include hydrogen chloride (HCl), carbon dioxide (CO2), and water (H2O).