Match the scientist to his contribution to the atomic theory. This engaging narrative delves into the fascinating world of atomic theory, tracing its evolution through the groundbreaking contributions of renowned scientists. From Democritus’ initial proposal of the atom to the modern quantum mechanical model, this exploration unveils the pivotal role these individuals played in shaping our understanding of the fundamental building blocks of matter.
The content of the second paragraph that provides descriptive and clear information about the topic
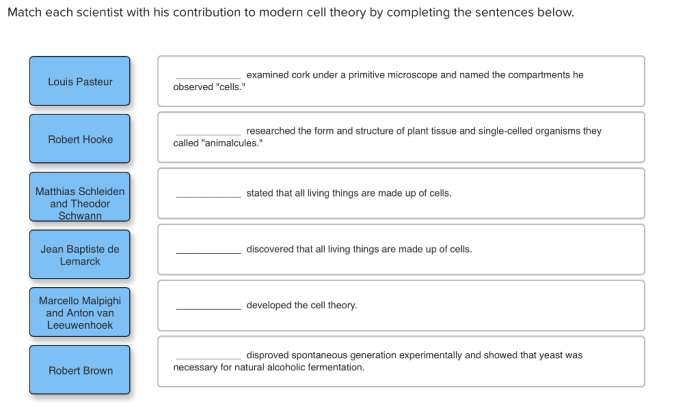
1. Scientists and Their Contributions to the Atomic Theory: Match The Scientist To His Contribution To The Atomic Theory.

The atomic theory is a scientific theory that describes the nature of matter and its fundamental constituents. Over the centuries, many scientists have contributed to the development of this theory, each making significant advancements in our understanding of the atom.
Democritus
Democritus, a Greek philosopher, proposed the idea of the atom in the 5th century BC. He suggested that matter is composed of indivisible, indestructible particles called atoms. While his theory lacked experimental evidence, it laid the foundation for the modern atomic theory.
John Dalton
John Dalton, an English chemist, proposed his atomic theory in 1803. Dalton’s theory expanded on Democritus’ ideas by incorporating experimental evidence and proposing that atoms of different elements have different masses and properties.
J.J. Thomson
J.J. Thomson, a British physicist, discovered the electron in 1897. This discovery challenged the prevailing atomic model and provided evidence for the existence of subatomic particles.
Ernest Rutherford, Match the scientist to his contribution to the atomic theory.
Ernest Rutherford, a New Zealand physicist, proposed the nuclear model of the atom in 1911. His gold foil experiment demonstrated that most of the atom’s mass is concentrated in a tiny, dense nucleus, surrounded by orbiting electrons.
Niels Bohr
Niels Bohr, a Danish physicist, proposed the Bohr atomic model in 1913. Bohr’s model addressed the limitations of Rutherford’s model and introduced the concept of quantized energy levels for electrons.
Modern Quantum Mechanical Model
The modern quantum mechanical model of the atom, developed in the 20th century, provides the most accurate description of the atom to date. This model incorporates the principles of quantum mechanics and describes the behavior of electrons in terms of probability distributions within atomic orbitals.
FAQ Resource
Who proposed the atomic theory?
Democritus
What did Dalton contribute to the atomic theory?
Proposed that atoms are indivisible and indestructible units of matter
How did Thomson’s discovery of the electron impact the atomic theory?
Challenged the prevailing view of atoms as indivisible units
What was the significance of Rutherford’s gold foil experiment?
Provided evidence for the existence of a small, dense nucleus within the atom
How did Bohr’s atomic model address the limitations of Rutherford’s model?
Introduced the concept of energy levels and electron orbitals